Understanding Electrochemical CO2 Reduction Selectivity of Cu Binary Alloys from Electronic Structure Descriptors
Abstract
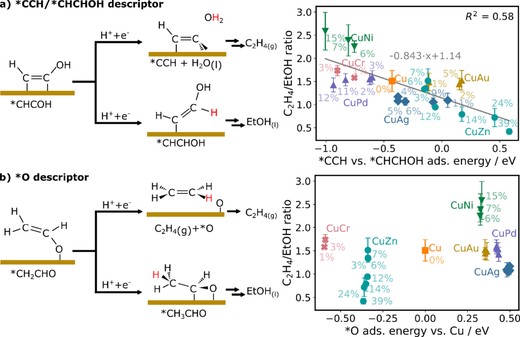
We investigate the electrochemical reduction of carbon dioxide (CO2RR)using Cu-M (M=Ni, Pd, Ag, Au, Zn, Cr) binary alloys. Through experimental and theoretical analysis, we explore the structural and electronic properties of these alloys and their impact on CO2RR product selectivity. Our findings reveal that the d-band center of Cu-M alloys serves as a crucial descriptor, influencing product distribution. As the d-band center increases, more CO is produced, while the formation of C2 products(ethylene and ethanol) follows a volcano-like trend. We also observe a linear scaling relationship between the d-band center and ethylene/ethanol selectivity, providing opportunities for fine tuning product selectivity. The observation is explained by theoretical calculations that suggest that the d-band center effectively changes the relative binding of *CCH vs *CHCHOH, there by controlling product selectivity into ethylene vs ethanol formation. We also find that this d-band center dependence is what makes the *CCH vs *CHCHOH adsorption energy,when properly corrected for alloy composition-dependencies, the most reliable theoretically accessible descriptor for ethylene/ethanol selectivity,while the *O adsorption energy does not show a clear correlation with experimental results.This study enhances our understanding of CO2RR catalysis and offers guidelines for designing Cu-based alloy electrocatalysts with improved activity and selectivity for sustainable CO2 conversion.