Spatiotemporal Nitric Oxide Modulation via Electrochemical Platform to Profile Tumor Cell Response
Abstract
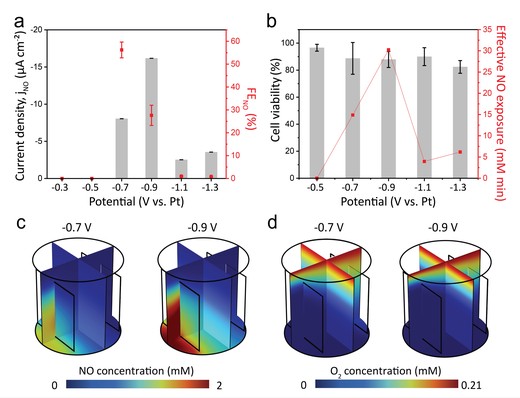
Nitric oxide (NO) is a gaseous molecule intricately implicated in oncologic processes, encompassing the modulation of angiogenesis and instigating apoptosis. Investigation of the antitumor effects of NO is currently underway, necessitating a detailed understanding of its cellular-level reactions. Regulating the behavior of radical NO species has been a significant challenge, primarily due to its instability in aqueous environments by rapid O2-induced degradation. In this study, we devised an electrochemical platform to investigate the cellular responses to reactive gaseous molecules. Our designed platform precisely controlled the NO flux and diffusion rates of NO to tumor cells. COMSOL Multiphysics calculations based on diffusion and reaction kinetics were conducted to simulate the behavior of electrochemically generated NO. We discerned that the effective distance, NO flux, and electrolysis duration are pivotal factors governing cellular response by NO.