Thermal Transformation of Molecular Ni2+–N4 Sites for Enhanced CO2 Electroreduction Activity
Abstract
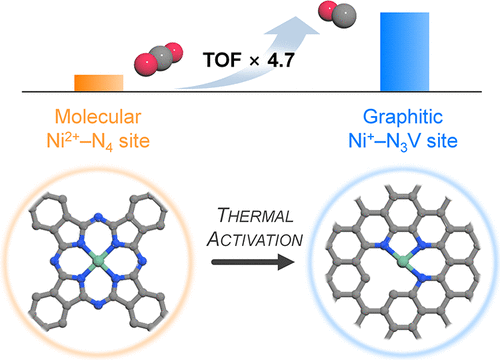
Atomically dispersed nickel sites complexed on nitrogen-doped carbon (Ni–N/C) have demonstrated considerable activity for the selective electrochemical carbon dioxide reduction reaction (CO2RR) to CO. However, the high-temperature treatment typically involved during the activation of Ni–N/C catalysts makes the origin of the high activity elusive. In this work, Ni(II) phthalocyanine molecules grafted on carbon nanotube (NiPc/CNT) and heat-treated NiPc/CNT (H-NiPc/CNT) are exploited as model catalysts to investigate the impact of thermal activation on the structure of active site and CO2RR activity. H-NiPc/CNT exhibits ~4.7-fold higher turnover frequency for CO2RR to CO in comparison to NiPc/CNT. Extended X-ray absorption fine structure analysis and density functional theory (DFT) calculations reveal that the heat treatment transforms molecular Ni2+–N4 sites of NiPc into Ni+–N3V (V: vacancy) and Ni+–N3 sites incorporated in the graphene lattice that concomitantly involves a breakage of Ni–N bonding, shrinkage in the Ni–N–C local structure, and decrease in the oxidation state of the Ni center from +2 to +1. DFT calculations combined with micro-kinetic modeling suggest that the Ni–N3V site appears to be responsible for the high CO2RR activity because of its lower barrier for the formation of *COOH intermediate and optimum *CO binding energy. In situ/operando X-ray absorption spectroscopy analyses further corroborate the importance of reduced Ni+ species in boosting the CO2RR activity.