Boosting Electrochemical CO2 Reduction to Methane via Tuning Oxygen Vacancy Concentration and Surface Termination on a Copper/Ceria Catalyst
Abstract
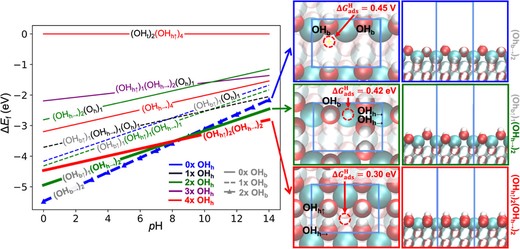
Metal oxides are a promising material for designing highly active and selective catalysts for the electrochemical reduction of carbon dioxide (CO2RR). Here, we designed a Cu/ceria catalyst with high selectivity of methane production at single-atomic Cu active sites. Using this, we report favorable design concepts that push the product selectivity of methane formation by combining detailed structural analysis, density functional theory (DFT), in situ Raman spectroscopy, and electrochemical measurements. We demonstrate that a higher concentration of oxygen vacancies on the catalyst surface, resulting from more available Cu+ sites, enables high selectivity for methane formation during CO2RR and can be controlled by the calcination temperature. The DFT calculation and in situ Raman studies indicate that pH controls the surface termination; a more alkaline pH generates hydroxylated surface motifs with more active sites for the hydrogen evolution reaction. These findings provide insights into designing an efficient metal oxide electrocatalyst by controlling the atomic structure via the reaction environment and synthesis conditions.