Electric field mediated selectivity switching of electrochemical CO2 reduction from formate to CO on carbon supported Sn
Abstract
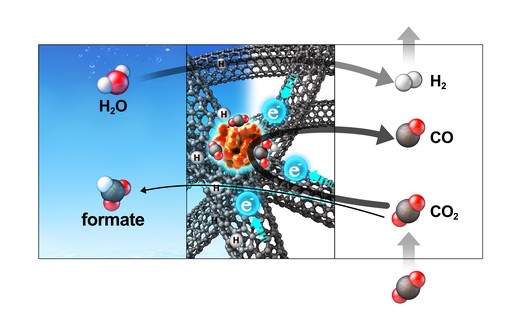
Decades of electrochemical CO2 reduction research have led to established rules about the product selectivity i.e. bare Tin yields formic acid as the main product. Here, we present Sn nanoparticles supported on carbon nanotubes in a hollow fiber (Sn-CHF) which produce CO with 10 times higher selectivity than formate. Density functional theory calculations reveal that a strong interfacial field induced by the carbon support enhances the rate-limiting CO2 adsorption and thus CO production on Sn nanoparticles whereas the field-insensitive formate and hydrogen production routes were completely suppressed and occurred mainly from carbon sites. Modification of the interfacial electric field via exchange of the electrolyte-containing cation from Li+ to Cs+ induces an unprecedented two orders of magnitude change in the CO current, while not changing the other products. This work demonstrates how electrochemical selectivity rules can be modulated by controlling the interfacial field, thus opening up new windows for electrocatalyst design.