Design of less than 1 nm Scale Spaces on SnO2 Nanoparticles for High‐Performance Electrochemical CO2 Reduction
Abstract
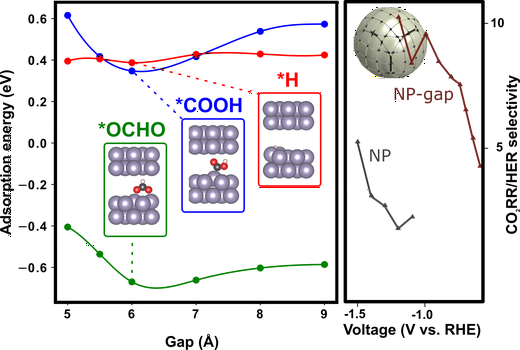
Electrochemical carbon dioxide reduction reaction (CO2RR) is a promising approach to mitigate CO2 concentration and generate carbon feedstock. Recently, the (sub-)nanometer design of catalyst structures has been revealed as an efficient means to control the reaction process through the local reaction environment. Herein, the synthesis of a novel tin oxide (SnOx) nanoparticle (NP) catalyst with highly controlled sub-nanoscale interplanar gaps of widths <1 nm (SnOx NP-s) is reported via the lithium electrochemical tuning (LiET) method. Transmission electron microscopy (TEM) and 3D-tomo-scanning TEM (STEM) analysis confirm the presence of a distinct segmentation pattern and the newly engineered interparticle confined space in the SnOx NP-s. The catalyst exhibits a significant increase in CO2RR versus hydrogen evolution selectivity by a factor of $≈$5 with 20% higher formate selectivity relative to pristine SnO2 NPs at ?1.2 VRHE. Density functional theory calculations and cation-size-dependent experiments indicate that this is attributable to a gap-stabilization of the rate-limiting *OCHO and *COOH intermediates, the formation of which is driven by the interfacial electric field. Moreover, the SnOx NP-s exhibits stable performance during CO2RR over 50 h. These results highlight the potential of controlled atomic spaces in directing electrochemical reaction selectivity and the design of highly optimized catalytic materials.