Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst
Abstract
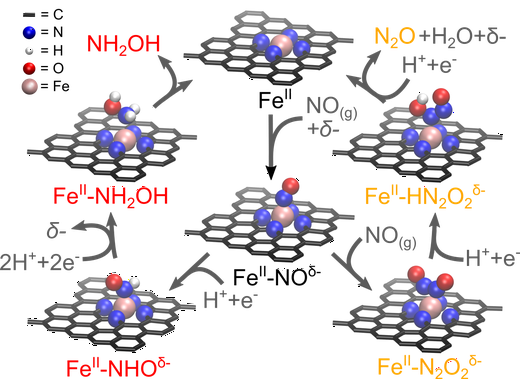
Electrocatalytic conversion of nitrogen oxides to value-added chemicals is a promising strategy for mitigating the human-caused unbalance of the global nitrogen-cycle, but controlling product selectivity remains a great challenge. Here we show iron–nitrogen-doped carbon as an efficient and durable electrocatalyst for selective nitric oxide reduction into hydroxylamine. Using in operando spectroscopic techniques, the catalytic site is identified as isolated ferrous moieties, at which the rate for hydroxylamine production increases in a super-Nernstian way upon pH decrease. Computational multiscale modelling attributes the origin of unconventional pH dependence to the redox active (non-innocent) property of NO. This makes the rate-limiting NO adsorbate state more sensitive to surface charge which varies with the pH-dependent overpotential. Guided by these fundamental insights, we achieve a Faradaic efficiency of 71% and an unprecedented production rate of 215 $μ$mol cm−2 h−1 at a short-circuit mode in a flow-type fuel cell without significant catalytic deactivation over 50 h operation. Electrocatalytic conversion of nitrogen oxides to value-added chemicals is a promising strategy for mitigating the imbalance in the global nitrogen cycle. Here, the authors present iron–nitrogen-doped carbon as an efficient and durable electrocatalyst for selective nitric oxide reduction to hydroxylamine.