Active and stable PtP2-based electrocatalysts solve the phosphate poisoning issue of high temperature fuel cells
Abstract
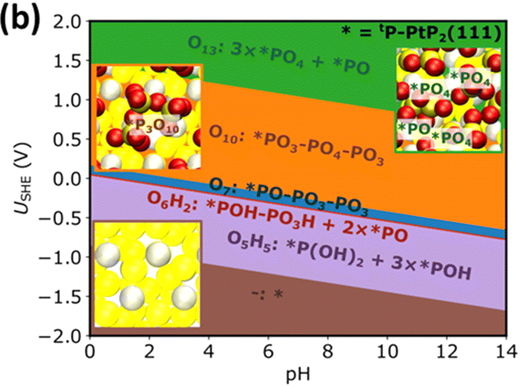
Platinum (Pt) loaded over a carbon support is known to be the best and most effective electrocatalyst for the oxygen reduction reaction (ORR). However, given its high surface energy, it tends to lose its catalytic activity after an unacceptably short period of usage. The stability of Pt has always been a bottleneck in commercializing the polymer electrolyte membrane fuel cell (PEMFC). In high temperature-polymer electrolyte membrane fuel cells (HT-PEMFCs), the activity loss has been traced back to the chemisorption of phosphate anions, which irreversibly poison Pt active sites. Herein, we present an alternative Pt phosphide-based (PtP2/C) electrocatalyst for application under high temperature conditions. The prepared PtP2/C catalyst shows surprisingly excellent long-term stability and high catalytic activity in phosphoric acid. From density functional theory (DFT) calculations, we found this to be related to the oxyphilicity of the P atoms which under reaction conditions form a protective phosphorus oxide film that also binds phosphoric acid more strongly than Pt sites. Thus-protected Pt sites, in particular those from P defects, are predicted to be highly active for the ORR. The improved stability is also the result of a better oxidation resistance of the carbon support in the presence of PtP2.