Tuning the C1/C2 Selectivity of Electrochemical CO2 Reduction on Cu-CeO2 Nanorods by Oxidation State Control
Abstract
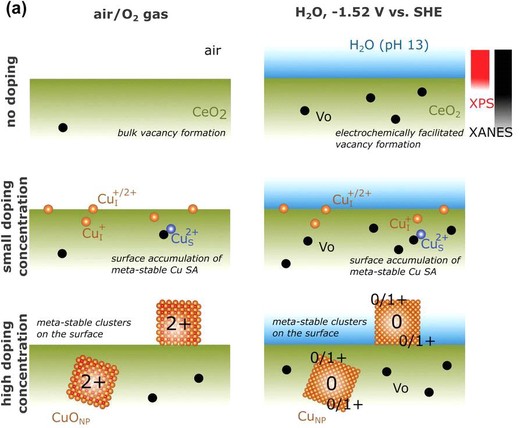
Ceria (CeO2) is one of the most extensively used rare earth oxides. Recently, it has been used as a support material for metal catalysts for electrochemical energy conversion. However, to date, the nature of metal/CeO2 interfaces and their impact on electrochemical processes remains unclear. Here, a Cu-CeO2 nanorod electrochemical CO2 reduction catalyst is presented. Using operando analysis and computational techniques, it is found that, on the application of a reductive electrochemical potential, Cu undergoes an abrupt change in solubility in the ceria matrix converting from less stable randomly dissolved single atomic Cu2+ ions to (Cu0,Cu1+) nanoclusters. Unlike single atomic Cu, which produces C1 products as the main product during electrochemical CO2 reduction, the coexistence of (Cu0,Cu1+) clusters lowers the energy barrier for C-C coupling and enables the selective production of C2+ hydrocarbons. As a result, the coexistence of (Cu0,Cu1+) in the clusters at the Cu-ceria interface results in a C2+ partial current density/unit Cu weight 27 times that of a corresponding Cu-carbon catalyst under the same conditions.