Alkali Metal Ion Substituted Carboxymethyl Cellulose as Anode Polymeric Binders for Rapidly Chargeable Lithium-Ion Batteries
Abstract
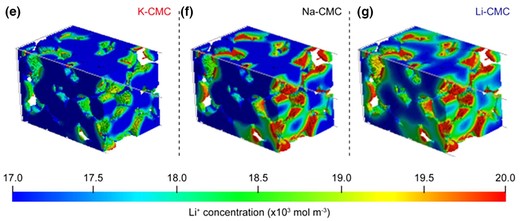
The increasing demand for short charging time on electric vehicles has motivated realization of fast chargeable lithium-ion batteries (LIBs). However, shortening charging time of LIBs is limited by Li+ intercalation process consisting of liquid-phase diffusion, de-solvation, SEI crossing, and solid-phase diffusion. Herein, we propose a new strategy to accelerate de-solvation step through control of interaction between polymeric binder and solvent-Li+ complexes. For this purpose, three alkali metal ions (Li+, Na+, and K+) substituted carboxymethyl cellulose (Li-, Na-, and K-CMC) are prepared to examine the effects of metal ions on their performance. The lowest activation energy of de-solvation and the highest chemical diffusion coefficient were observed for Li-CMC. Specifically, Li-CMC cell with a capacity of 3 mAh cm-2 could be charged to >95% in 10 min, while a value above >85% was observed after 150 cycles. Thus, the presented approach holds great promise for the realization of fast charging.