Abstract
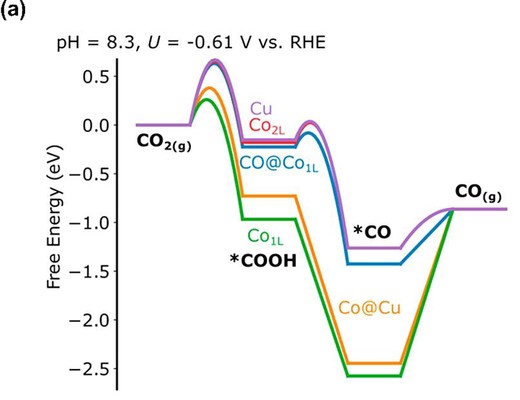
The development of Cu-based catalysts for electrochemical CO2 reduction reaction (CO2RR) with stronger CO-binding elements had been unsuccessful in improving multicarbon production from the CO2RR due to CO-poisoning. Here, we discover that trace doping levels of Co atoms in Cu, termed CoCu single-atom alloy (SAA), achieve up to twice the formation rate of CO as compared to bare Cu and further demonstrate a high jC2H4 of 282 mA cm2 at −1.01 VRHE in a neutral electrolyte. From DFT calculations, Cu sites neighboring CO-poisoned Co atomic sites accelerate CO2-to-CO conversion and enhance the coverage of *CO intermediates required for the formation of multicarbon products. Furthermore, CoCu SAA also exhibits active sites that favor the deoxygenation of *HOCCH, which increases the selectivity toward ethylene over ethanol. Ultimately, CoCu SAA can simultaneously boost the formation of *CO intermediates and modulate the selectivity toward ethylene, resulting in one of the highest ethylene yields of 15.6%.