CO Cryo-sorption as a Surface-sensitive Spectroscopic Probe of the Active Site Density of Single-atom Catalysts
Abstract
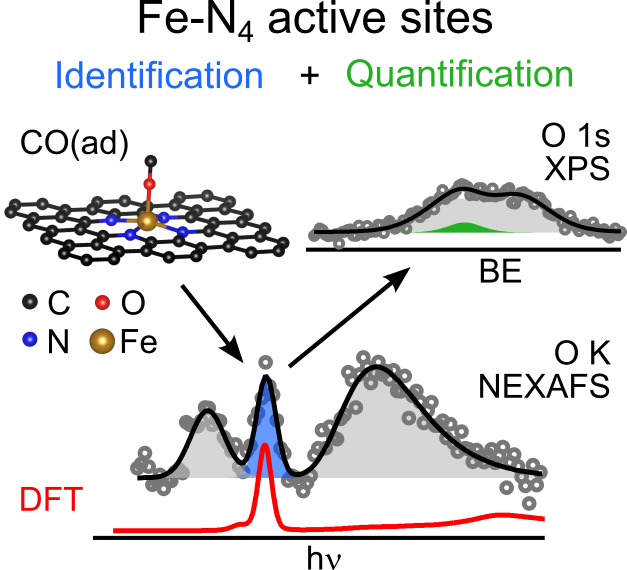
Quantifying the number of active sites is a crucial aspectin the performance evaluation of single metal-atom electrocatalysts. A possible realization is using adsorbing gas molecules that selectively bind to the single-atom transition metal and then probing their surface density using spectroscopic tools. Herein, using in situ X-ray photoelectron (XPS) and near edge X-ray absorption fine structure (NEXAFS) spectroscopy, we detect adsorbed CO gas molecules on a FeNC oxygen reduction single atom catalyst. Correlating XPS and NEXAFS, we develop a simple surface-and chemically-sensitive protocol to accurately and quickly quantify the active site density. Density functional theory-based X-ray spectra simulations reaffirm the assignment of the spectroscopic fingerprints of the CO molecules adsorbedat Fe-N4-C sites, and provide additional unexpected structural insights about the active site needed to explain the low-temperature CO adsorption. Our work represents an important step towards an accurate quantitative catalytic performance evaluation, and thus towards developing reliable material design principles and catalysts.